◆ A Lifecycle Approach for Analytical Instrument and Systems Qualification (26-Aug-20 ECA)
In July 2018, the ECA AQCG published version one of its guideline on Analytical Procedure Lifecycle Management (APLM) in support of the development of USP <1220>¹ ,² and the ICH revision of Q2(R2) and the newly proposed Q14. The proposed overall lifecycle is shown in Figure 1.
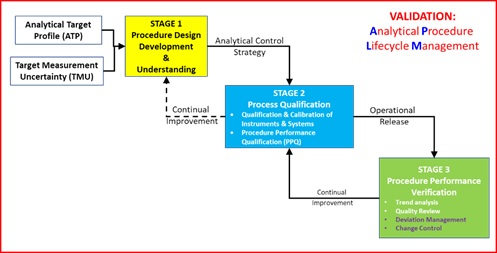
Figure 1 Lifecycle model for Analytical Procedure Lifecycle ³
As can be seen, validation is no longer a discrete activity but a journey. As part of that journey, the first aspect of Stage 2 Process Qualification requires Qualification and Calibration of Instruments and Systems as one prerequisite for Procedure Performance Qualification. The Analytical Process, conducted within a Pharmaceutical Quality Management System, provides reliable, traceable and secure reportable values and is shown in Figure 2 together with the relationship to Stage 2 of the APLM.
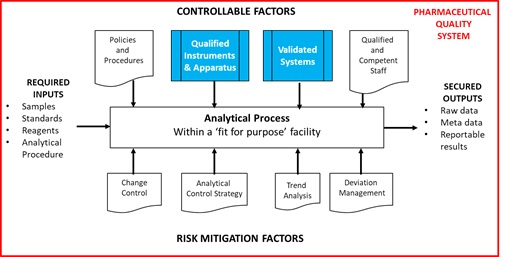
Figure 2 The Analytical Process
As an extension of our APLM guideline, the AQCG is in the process of drafting an equivalent lifecycle for the Qualification and Calibration of Instruments and Systems.
The proposed lifecycle model is shown in Figure 3 and will be discussed in detail in the forthcoming Guideline.
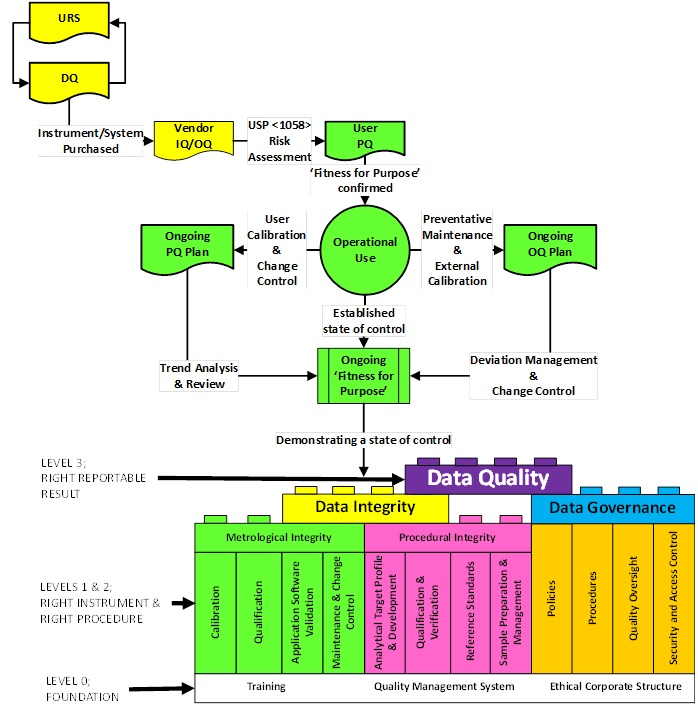
Figure 3 Proposed AISQ lifecycle
Currently there is much interest on metrological integrity and is the main focus of this short article. However elements of Data Quality ie Integrity and Governance have been addressed in Version 2 of the ECA GMP data Governance and Data Integrity Guideline of January 2018.
From a metrological integrity point of view, the pharmacopoeias, particularly the USP and Pharm. Eur., contain standards of performance required by analytical instruments and systems to demonstrate ‘fitness for purpose’.
However, there are a diversity of definitions for Calibration, Qualification, Verification, and Validation within the USP General Chapters which need to be harmonized to assist understanding and prevent user confusion. This is particularly true for the differentiation of holistic and modular calibration standards and the expression of acceptance criteria for establishing ‘fitness for purpose’. For example the calibration of spectrometers4,5
A recent paper6 on this topic, discusses these issues as well as the regulatory requirements and provides a calibration and qualification process flow model, involving both users and vendors as shown in Figure 4.
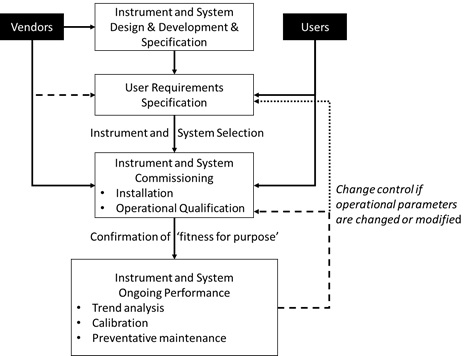
Figure 4 Generic flow for the calibration and qualification lifecycle
The topic of analytical instrument qualification will also be discussed during the Live Online Training Analytical Instrument Qualification on 15 October.
Source:
Dr Christopher Burgess
Chairman of the ECA Analytical Quality Control Group
¹ Lifecycle Management of Analytical Procedures: Method Development, Procedure Performance Qualification, and Procedure Performance Verification; Pharm. Forum 39(5) 2013,
² Proposed New USP General Chapter: The Analytical Procedure Lifecycle <1220>, Pharm. Forum. 43(1) 2017
³ ECA AQCG Guideline; LABORATORY DATA MANAGEMENT GUIDANCE, Analytical Procedure Lifecycle Management, (APLM), Version 1, July 2018
4 C. Burgess, “Calibration of instruments: is your UV spectrometer accurate enough?”, Pharm. Tech. 38(1) (2014)
5 C Burgess, JP Hammond; Is you spectrometer in calibration?, Spectroscopy Europe, 28(2 2016
6 C Burgess, A Life Cycle Approach to the Calibration and Qualification of Analytical Instruments and Systems to Establish ?Fitness for Purpose? for Pharmacopeial Purposes, Pharm. Forum 46(4) 2020
◆ What are FDA’s Requirements for Pharmaceutical Quality – YouTube Videos give Answers (23-Sep-20 ECA)
The FDA presented its requirements for pharmaceutical quality, including quality assurance aspects in a webinar. This webinar focused on stakeholders in the pharmaceutical supply chain worldwide. The presentations are available as YouTube videos.
According to the FDA, the view on pharmaceutical quality should be uniform. In the age of globalisation where there are many parties involved in the supply chain of pharmaceuticals. In order to also address stakeholders who are spread over different locations worldwide and therefore work in different time zones, the FDA has made the slides of the lectures on pharmaceutical quality and also the lectures themselves available as YouTube videos.
The target groups mentioned are
There are a total of 12 presentations, which also contain data on inspections and inspection deficiencies, for example, but also deal with topics relevant to authorisation such as “Changes”.
You can find access to the slides and videos under “A Pharmaceutical Quality Webinar for Global Stakeholders” on the FDA Website.
Copyright © 2019 NAGANO SCIENCE CO., LTD. All Rights Reserved